1. The diagram above shows the repeating groups of atoms that make up two samples. Will the
properties of the two samples likely be the same or different? (Examples of properties are smell,
color, and the temperature at which a substance melts.)
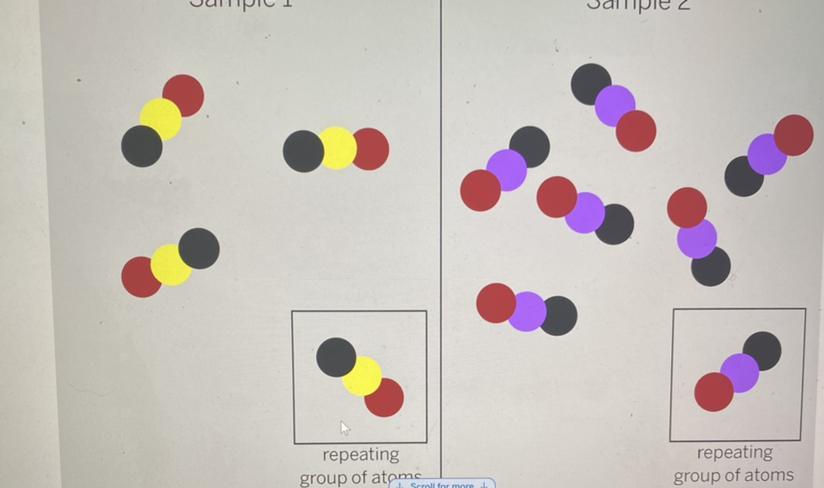
Answers
Answer:
Will likely be the same
Explanation:
We can see in both pictures there is a black molecule and a red molecule. However, we also have a purple molecule in one image and a yellow in the other. It would LIKELY be the same because we have more of the same molecules then more different molecules. Hope this helps
Related Questions
Calculate:for each object, substitute the values you know into the gravitational potential energy equation to solve for weight. Record each object's weight in the fourth column.
Answers
Answere:No sé esto jeje lo siento no soy tonta pero simplemente no sé esto
Explanation:
Use the diagram below to answer the question.
An Energy Relationship in an Ecosystem
grass
mouse
Which statement best describes the energy relationship shown between the organisms?
Both produce their own energy.
b Only the mice need energy to survive.
They compete for the same source of energy.
Energy flows from the producer to the consumer.
Answers
Answer:Where is the diagram
Explanation:
Write the equilibrium expressions for each of the following equilibria:
a. 2 Ba + O2 ⇌ 2 BaO
b. 2 Mg + O2 ⇌ 2MgO
c. P4 + 5 O2 ⇌ P4O10
Answers
Answer:
a.
[tex]K=\frac{[BaO]^2}{[Ba]^2[O_2]}[/tex]
b.
[tex]K=\frac{[MgO]^2}{[Mg]^2[O_2]}[/tex]
c.
[tex]K=\frac{[P_4O_{10}]^2}{[P_4][O_2]^5}[/tex]
Explanation:
Hello!
In this case, since the equilibrium expression is set up by dividing the products over the reactants and powering to the stoichiometric coefficient, we can proceed as follows:
a. 2 Ba + O2 ⇌ 2 BaO.
[tex]K=\frac{[BaO]^2}{[Ba]^2[O_2]}[/tex]
b. 2 Mg + O2 ⇌ 2MgO
[tex]K=\frac{[MgO]^2}{[Mg]^2[O_2]}[/tex]
c. P4 + 5 O2 ⇌ P4O10
[tex]K=\frac{[P_4O_{10}]^2}{[P_4][O_2]^5}[/tex]
Best regards.
What is the pH of a solution with a (H+] = 0.80 M? *Please round your answer to the appropriate number of significant figures. Your answer can be in standard notatic "e" in place of x10.*
Answers
Answer:
The pH of a solution with a (H+] = 0.80 M is [tex]9.6 e^{-2}[/tex]
Explanation:
As we know
pH = -[log H+]
Substituting the value of H+ ion concentration in the above equation, we get -
pH = -log [0.80]
pH [tex]= -[-0.096] = 0.096 = 9.6 e^{-2}[/tex]
how many liters are in 4x10^23 atoms of CO
Answers
Answer:
3.346*10^25
Explanation:
Hope this helps
OMG PLEASEEEE HELPPPPPPPPPPP!!!!!!!!!!!
Answers
Answer:
the second one
Explanation:
THE answers is b
Please help!!! What is electrolysis?
Answers
Explanation:
plzz tell me the ans even i also want to know plzzzz someone say ans
Answer:
electrolysis is a procedure that uses direct electric current to achieve an otherwise non-spontaneous chemical reaction and is important in the separation of elements from naturally occurring
5
Increasing the amount of current flowing through a wire strengthens
what? *
O magnetic field
O polarity
O electromagnetism
solenoid
Answers
Answer:
Increasing the current would strengthen the
electromagnetisn
why is atomic radi measured using the nuclues from 2 of the same atoms
Answers
Explanation:
Atomic radii is measured using the distance between nucleus of 2 atoms rather than the distance between the nucleus and outermost shell because:
- There is no clear/sharp boundary of the orbital. This is why it is called an electron cloud.
- Also, the exact location of the electron is not known. What is known is the probability of finding the electron there.
Due to this, it is not possible to measure the atomic radii precisely. That's why the distance between the nucleus of two atoms is used.
How much sugar can be dissolved of the 0.3 ml of water is heated to 1000C?
Answers
1. What two types of cells contain chloroplasts?
Answers
Answer:
plant cells and eukroyatic algae
what are the chemical properties of acids
Answers
Answer:
Acids are sour in taste. Acids react with carbonates and hydrogen carbonates to form a salt, water, and carbon dioxide gas. Extremely active metals such as Potassium (K), Calcium (Ca), Sodium (Na), etc tend to explode when combined with acids. Weak Acids like Carbonic Acid doesn't act with any metal at all.
I copied and pasted
but I hope the info helps
Humans become part of the carbon cycle when they
A. breathe in carbon dioxide,
B. release carbon as a waste product,
C. produce new carbon compounds in their cells,
Answers
Answer:
Human activities have a tremendous impact on the carbon cycle
Explanation:
Burning fossil fuels, changing land use, and using limestone to make concrete all transfer significant quantities of carbon into the atmosphere. ... The ocean absorbs much of the carbon dioxide that is released from burning fossil fuels.
do all energy transfers lead to a phase change
Answers
Answer: Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system
Explanation:
What is the charge of cobalt in CoCl2?
Answers
Answer:
The oxidation number of cobalt is +2.
Please help me with this question tyy
Answers
Oh sheesh girl.. this is tricky.. but I can try:)
Between the chemical reaction of the chemicals and the label on the bottle the ions from in the radioactive product contained in the bottle separates the label from the surface. Im guessing that due to the "radioactive waste" in the bottle that there are components of thermal heat, that may also have an effect on the "label". So when talking about the reaction we have a chemical and thermal reaction happening.
I tried... and im sorry if it dosen't make sense... +_=||
have a wonderful day :)
Someone please help me with this I dont know if this is correct
Answers
Answer:
Yes its correct
Explanation:
Protons + Nuetrons
Answer: Go with your gut cos
Explanation:
How many grams of hydrogen reacts with 32 g of oxygen to produce 34 g of hydrogen peroxide
Answers
Answer:
2 grams.
Explanation:
H2 + O2 ---> H2O2
Using molar masses:
2*1 g hydrogen reacts with 2*16 g oxygen.
so 2g H2 reacts with 32 g O2.
What is the meaning of the word neutral?
Answers
Explanation:
having no strongly marked or positive characteristics or features.
In an experiment, students were instructed to equally heat a sample of soil and a sample of water with heat lamps and then measure the temperature of each sample every minute for five minutes. The results are shown in the table.Which of the following predictions is BEST supported by the data?
Answers
Answer:
Explanation:
D. i had this on my science test!!!
Answer:
D
Explanation:
yup its D
Study the chemical reaction below and answer the questions that follow: 8Al + 3Fe₃O₄ → 4Al₂O₃ + 9Fe How many moles of iron would be produced if 4.0 moles of aluminum reacts with an excess of Fe₃O₄?
A. 4.0 moles
B. 4.5 moles
C. 8.0 moles
D. 9.0 moles
Answers
Answer: the answewr is A. hope this helps!
Explanation:
For a theoretical yield of 5.52 g and percent
yield of 51.7877%, calculate the actual vield
for a chemical reaction.
Answer in units of g.
Answers
Answer:
2.85868104g
Explanation:
Actual yield
Theoretical yield x 100 = 51.7877%
So, do the inverse and you get 2.85868104g
mi
Which two types of energy are formed by the transformation shown in the
photo?
A. Thermal energy - electrical energy
B. Chemical energy - electromagnetic energy
MC. Thermal energy -- chemical energy
D. Chemical energy - thermal energy
Answers
Answer:
A Thermal Energy and Electrical Energy
Explanation:
First it goes thermal then it transposed to electricity
Answer:
c and A
Explanation:
convert 0.881 mol N2 at STP to volume in liters
Answers
Answer:
19.7 L N2
Explanation:
The volume of the nitrogen gas at STP is equal to 19.72 L.
What is the ideal gas equation?The ideal gas equation can be defined as an equation that describes the behavior of an ideal gas. This equation gives by the product of the volume and pressure is equal to the product of the moles of gas, gas constant, and absolute temperature.
The mathematical form of the ideal gas law is given as follows:
PV = nRT
Where n is the moles, V is the volume, R is the universal gas constant and P is the pressure.
The temperature of the N₂ , T = 0° C = 273 K
The pressure of the gas at STP, P = 1 atm
The value of the universal gas constant, R = 0.082 atm L/K mol
The number of moles of N₂, n = 0.881 mol
Fill in the values n, R, P, and T in the equation, and we get:
1 × V = 0.881 × 0.082 × 273
V = 19.72 L
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ2
Air with less than 19.5% oxygen cannot support human life. How many moles of oxygen would the caisson if the percent of oxygen in the air dropped to 19.4%.
Answers
need the names for these asap please
Answers
Answer:
3_ethylpentane
4_ethylheptane
1_pentene
3_hexene
What is the chemical formula for Magnesium and Phosphorus as a compound?
1- Mg2 P5
2- Mg2 P3
3- Mg3 P2
4- Mg5 P3
Answers
The answer is Mg3 glad I helped
1CaC2 + 2O2 ---> 1Ca+2CO2
I need the mole to mole ratio.
Answers
Solid potassium chloride is obtained by the reaction of solid potassium and chlorine gas.
Write a balanced chemical equation for this reaction.
Answers
Answer:
2 K(s) + Cl₂ (g) --> 2 KCl(s)
Explanation:
Potassium will just be K
Chlorine gas is part of BrIClHOF, which are diatomic gasses. So Cl₂
Looking at the periodic table potassium K has an ion charge of +1 and chlorine Cl has an ion charge of -1, so in a balanced compound they will be written as KCl
Balancing the amounts of each will lead to 2 K(s) + Cl₂ (g) --> 2 KCl(s)
How many milliliters of 0.25M H2SO4 can be prepared from 57 mL of a 3.0M solution of H2SO4?
Answers
Answer:
Why ? Because 1 molecule of H2SO4 gives 2 H+ ions per molecule while only one H+ ion is required to neutralize 1 molecule of KOH. So, 1 molecule of H2SO4 can neutralize 2 molecules of KOH. Hence, we would require 525 ml of 0.03 M H2SO4 to neutralize 525 ml of 0.06 M KOH. How will we prepare 525 ml of 0.03 M H2SO4 ?
Explanation:
Now, we have 0.025 M H2SO4 and we do not know how much volume we have.
We will use the standard N1 X V1 = N2 X V2 for this calculation.
N1=0.025 M; V1=unknown; N2=0.03 M and V2=525 ml.
So V1= (0.03 X 525)/(0.025) = 630 ml.
According to the molar concentration, 684 ml of 0.25 M H₂SO₄ can be prepared from 57 mL of a 3.0 M solution of H₂SO₄.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.In case of 2 solutions,it is calculated as, M₁V₁=M₂V₂ substitution gives V₁=3×57/0.25=684 ml.
Thus, 684 ml of 0.25 M H₂SO₄ can be prepared from 57 mL of a 3.0 M solution of H₂SO₄.
Learn more about molar concentration,here:
https://brainly.com/question/15532279
#SPJ3